Platform technology
Developing Therapies With no Compromise
Sagitta© biodegradable conjugation platforms are discovered to reinforce ideal performances in terms of the therapeutic index, pharmacokinetics and manufacturability.
Sagitta©
Developing Therapies With no Compromise
Sagitta© biodegradable conjugation platforms are discovered to reinforce ideal performances in terms of the therapeutic index, pharmacokinetics and manufacturability.
The lead candidate: RS-0139
The lead candidate: RS-0337
The lead candidate: RS-0139
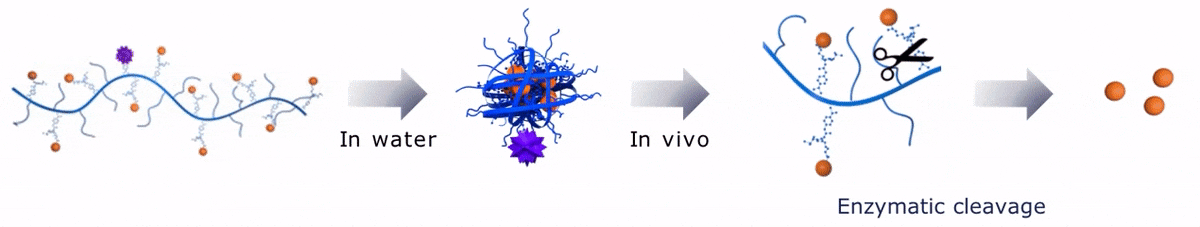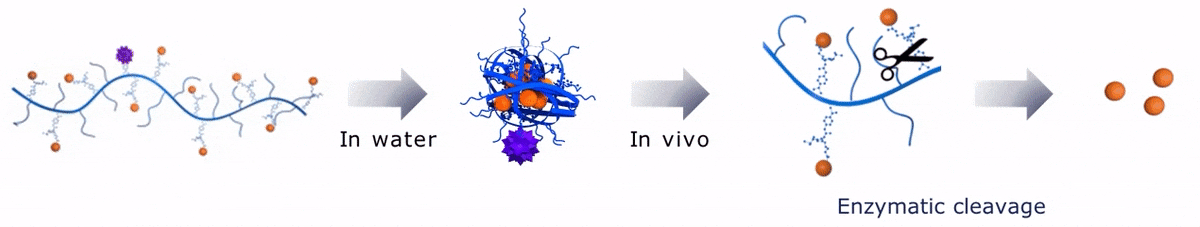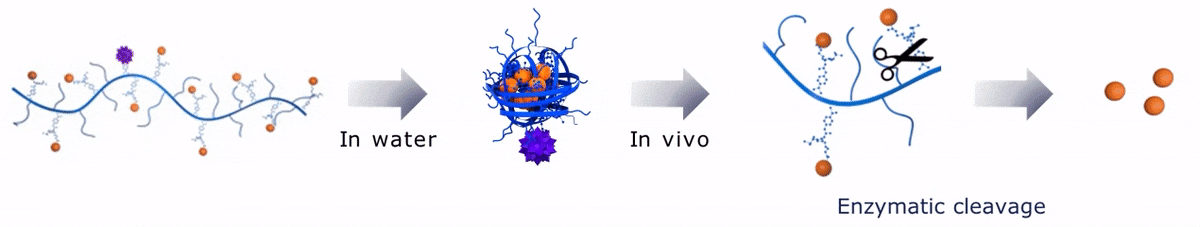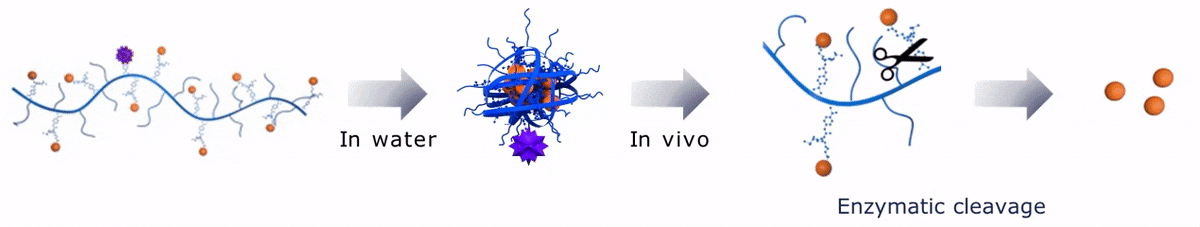
Upon contact with water, Sagitta© Bir backbone wraps around the payload, ensuring formation of a water soluble nanocarrier.
The lead candidate: RS-0337
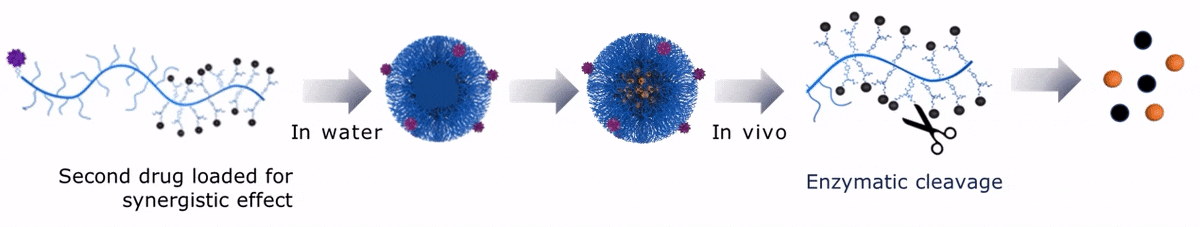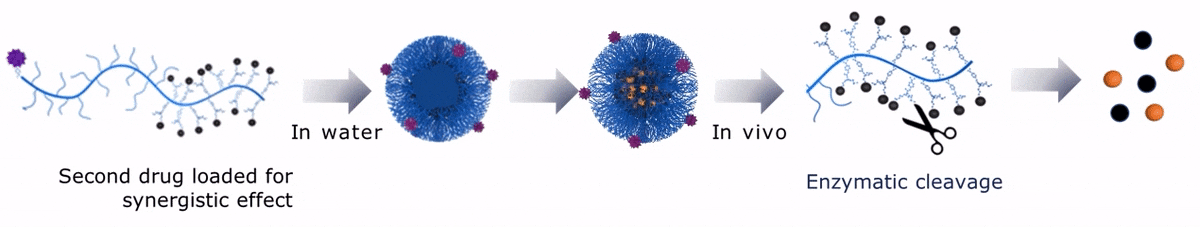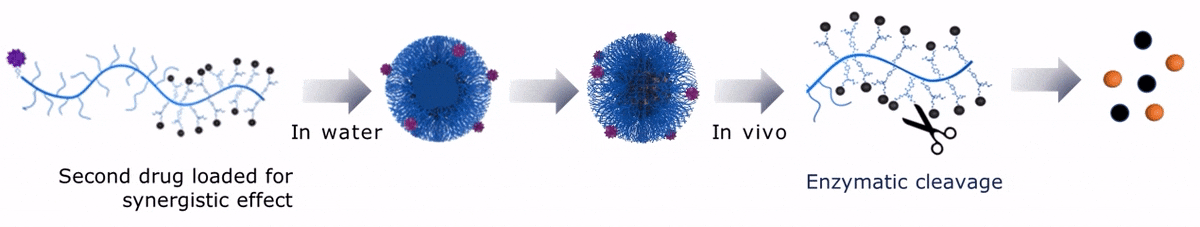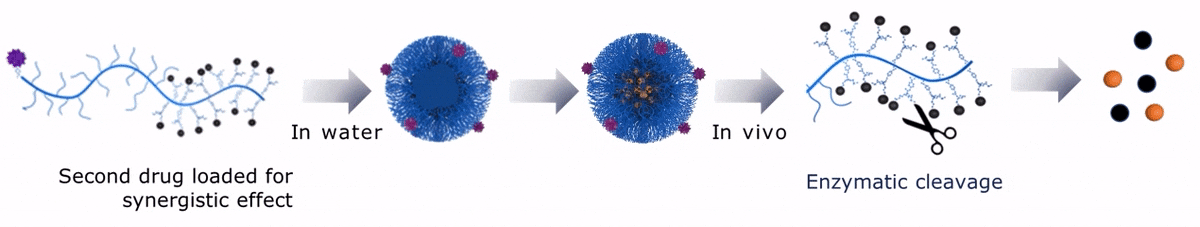 Upon contact with water, Sagitta© Dui forms a micellar nanoparticle, encapsulating the second payload.
Upon contact with water, Sagitta© Dui forms a micellar nanoparticle, encapsulating the second payload.
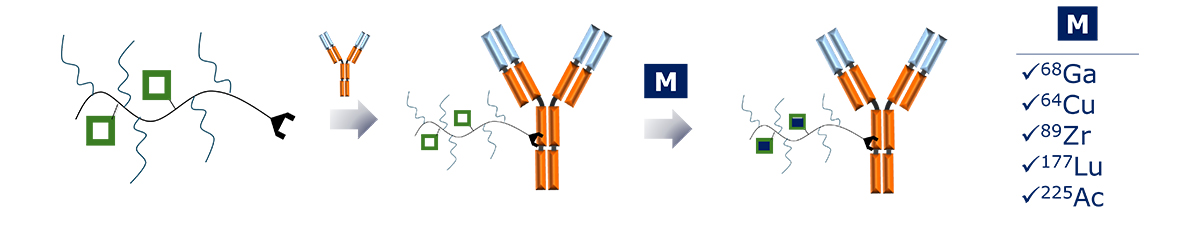 Sagitta© Radix’s design allows for precise targeting of disease sites via monoclonal antibodies (mAbs) or peptides for theranostic applications.
Sagitta© Radix’s design allows for precise targeting of disease sites via monoclonal antibodies (mAbs) or peptides for theranostic applications.
Therapeutic Index
The dual drug loading capacity of Sagitta© yields to a synergistic therapeutic effect. Sagitta© platform is compatible with a wide range of targeting ligands or mAbs as well as a diverse choice of drugs – ensuring highest efficacy and specificity.
Pharmacokinetics
Sagitta© platform significantly prolongs the circulation time compared to native API. The improvement in half-life translated across species and therefore poses predictable promise for later clinical stages.
Manufacturing
We manufacture our products at our own GMP certificated production facility for clinical batch lyophilized oncology drugs.
Efficacy & Specificity
Efficacy & Specificity
· Synergistic therapeutic effect ·
· Higher accumulation ·
Translatability
Translatability
· Predictable translation to human ·
Tolerability
Tolerability
· Enhanced Permeability & Retention effect ·
· Selective release ·
· Biologic stimuli driven release ·
Manufacturability
Manufacturability
· High purity ·
· Scalable for clinical GMP batches ·
HIGHER
THERAPEUTIC INDEX
The dual drug loading capacity of Sagitta© yields to a synergistic therapeutic effect. Sagitta© platform is compatible with a wide range of targeting ligands or mAbs as well as a diverse choice of drugs – ensuring highest efficacy and specificity.
IMPROVED
PHARMACOKINETICS
Sagitta© platform significantly prolongs the circulation time compared to native API. The improvement in half-life translated across species and therefore poses predictable promise for later clinical stages.
STRAIGHT-FORWARD
MANUFACTURING
We manufacture our products at our own GMP certificated production facility for clinical batch lyophilized oncology drugs.
Efficacy & Specificity
Efficacy & Specificity
· Synergistic therapeutic effect
· Higher accumulation
Tolerability
Tolerability
· Enhanced Permeability & Retention effect
· Selective release
· Biologic stimuli driven release
Translatability
Translatability
· Predictable translation to human
Manufacturability
Manufacturability
· High purity
· Scalable for clinical GMP batches
