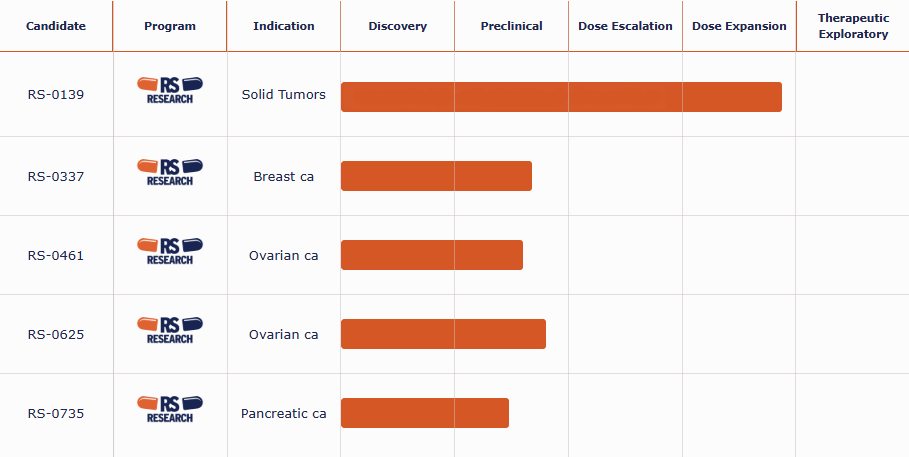
| Candidate | Program | Indication | Discovery | Preclinical | Dose Escalation | Dose Expansion | Therapeutic Exploratory |
|---|---|---|---|---|---|---|---|
| RS-0139 |  |
Solid Tumors | |||||
| RS-0337 |  |
Breast ca |
| ||||
| RS-0461 |  |
Ovarian ca |
| ||||
| RS-0625 |  |
Ovarian ca |
| ||||
| RS-0735 |  |
Pancreatic ca |
| ||||
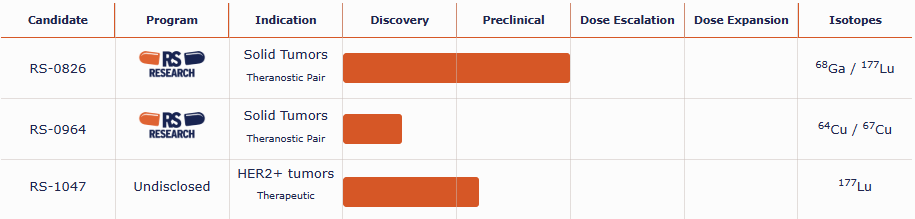
| Candidate | Program | Indication | Discovery | Preclinical | Dose Escalation | Dose Expansion | Isotopes |
|---|---|---|---|---|---|---|---|
| RS-0826 |  |
Solid Tumors Theranostic Pair |
68Ga / 177Lu | ||||
| RS-0964 |  |
Solid Tumors Theranostic Pair |
| 64Cu / 67Cu | |||
| RS-1047 | Undisclosed | HER2+ tumors Therapeutic |
| 177Lu | |||
We may request cookies to be set on your device. We use cookies to let us know when you visit our websites, how you interact with us, to enrich your user experience, and to customize your relationship with our website.
Click on the different category headings to find out more. You can also change some of your preferences. Note that blocking some types of cookies may impact your experience on our websites and the services we are able to offer.
These cookies collect information that is used either in aggregate form to help us understand how our website is being used or to help us customize our website for visitors in order to enhance their experience.
All informations which are collected by this way are anonymous.
If you do not want that we track your visit to our site you can disable tracking in your browser here:
We also use different external services like Google Webfonts and Google Maps. Since these providers may collect personal data like your IP address we allow you to block them here. Please be aware that this might heavily reduce the functionality and appearance of our site. Changes will take effect once you reload the page.
Google Webfont Settings:
Google Map Settings:
